Background: Tazemetostat, a first-in-class, oral enhancer of zeste homolog 2 (EZH2) inhibitor was recently approved by the US Food and Drug Administration in patients with relapsed/refractory (R/R) follicular lymphoma (FL) after demonstrating single-agent, antitumor activity in patients with wild-type (WT) or mutant (MT) EZH2. The PI3K inhibitors idelalisib, duvelisib, and copanlisib are indicated for third-line or later (3L+) treatment of R/R FL, but they are associated with safety concerns, and clinical studies with these agents did not include grade 3B or transformed FL. However, tazemetostat idelalisib, duvelisib, and copanlisib were all approved based on single-arm studies and have not been compared in head-to-head randomized trials for the treatment of 3L+ R/R FL. Here, we present an indirect treatment comparison (ITC) of tazemetostat with idelalisib, duvelisib, and copanlisib for the treatment of 3L+ R/R FL.
Methods: A systematic literature review identified clinical trial publications for idelalisib (DELTA), duvelisib (DYNAMO), and copanlisib (CHRONOS-1 Part B) for use in an ITC with tazemetostat for 3L+ treatment of R/R FL. Matching-adjusted indirect comparison (MAIC) methodology was selected as all comparator trials were single-arm and individual patient data (IPD) were available for the tazemetostat E7438-G000-101 trial (n=99). Three MAIC analyses were conducted by weighting individual patients treated with tazemetostat to baseline characteristics reported from each comparator trial. FL subpopulation baseline characteristics and outcomes data were available for matching idelalisib (n=72); full trial mixed-NHL populations were reported for duvelisib (n=129, 64% FL) and copanlisib (n=142, 73% FL). Baseline characteristics for matching-adjustment were chosen using clinical advice, an evaluation of prognostic factors associated with outcomes, and published data availability. Characteristics included: age, ECOG performance status, disease stage, histology (tumor grade, transformed FL), number of prior lines of treatment, prior stem cell therapy, and refractory status (to last therapy). Only the tazemetostat trial included patients with grade 3b or transformed FL, or recorded EZH2 mutation status. Safety and efficacy outcome definitions were similar across trials. Primary safety outcomes included risk of any grade ≥3 treatment-emergent adverse event (TEAE), any treatment-emergent serious adverse event (TESAE), and TEAEs leading to dose reduction, drug discontinuation, or interruption. The primary efficacy outcome was objective response rate (ORR), reported across all trials. Rates of individual grade ≥3 TEAEs also were compared. Limitations of such indirect methods include inability to account for any unmeasured covariates that may be effect modifiers, and reductions in the effective sample size.
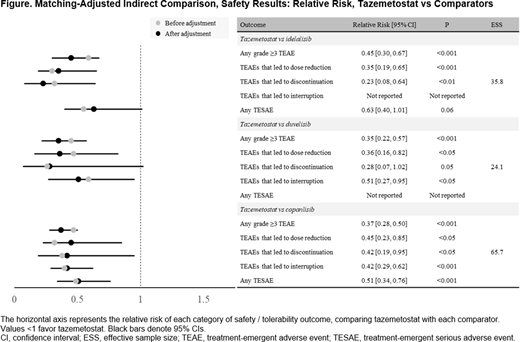
Results: After matching, all baseline characteristics were successfully balanced. Matched patients treated with tazemetostat had lower relative risk for all safety outcomes compared with all treatments, including any grade ≥3 TEAE (vs idelalisib: RR=0.45; vs duvelisib: RR=0.35; vs copanlisib: RR=0.37; all, P<0.001), any TESAE, and any TEAE leading to dose reduction, drug discontinuation, or interruption. These results were statistically significant (where comparator data were reported) for all but 2 safety outcomes (Figure). Several grade ≥3 TEAEs occurred at a significantly lower incidence with tazemetostat compared with matched patients treated with idelalisib, duvelisib, or copanlisib, including neutropenia (vs idelalisib: 3% vs 22%; vs duvelisib: 3% vs 25%; vs copanlisib: 4% vs 24%; all, P<0.05). The ORR was similar after matching for tazemetostat versus all treatments, with no statistically significant difference between therapies (vs idelalisib: 43% vs 56%, P=0.16; vs duvelisib: 48% vs 47%, P=0.91; vs copanlisib: 49% vs 61%, P=0.11).
Conclusion: More tolerable treatment options are needed for 3L+ treatment of R/R FL because patients in this setting are often elderly and have exhausted multiple prior lines of treatment. Results from this ITC indicate that, after adjusting for baseline population differences, tazemetostat addresses this unmet need, as it is associated with lower relative risk for safety outcomes versus idelalisib, duvelisib, or copanlisib while achieving similar efficacy outcomes.
Proudman:Analysis Group, Inc.: Consultancy. Nellesen:Analysis Group, Inc.: Consultancy. Gupta:Analysis Group, Inc.: Consultancy. Adib:Epizyme, Inc.: Consultancy; Alacrita: Current Employment. Yang:Epizyme, Inc.: Current Employment. Keith:Epizyme: Current Employment, Current equity holder in publicly-traded company. Mamlouk:Epizyme: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.